Tomorrow, Sarepta will be presenting updated data on the ability of their exon-skipping drug eteplirsen to produce dystrophin in 12 Duchenne Muscular Dystrophy (DMD) patients that have been treated in a phase I/II study now out over 3 years. They have rescored dystrophin data from previous muscle biopsies, and they have also conducted a 4th biopsy in a number of the patients that have been analyzed for dystrophin production. They also are expected to present updated 6 minute walk distance as well as other functional data. The interpretation of both 6 minute walk data & dystrophin data have been controversial so far, but here I will focus on the data Sarepta has reported so far on the ability of their drug to produce dystrophin - the protein missing in patients with DMD. First, some of the basics that those familiar with the approach can skip over.
The Basics: Dystrophin & Exon Skipping
Dystrophin is a protein important for muscle fiber function & stability, and is the causal gene mutated in DMD. Many patients have deletions (or duplications) of segments of the gene, called exons, the parts of the gene that encode the segments of RNA that get stitched together (splicing) to make the final RNA product. The RNA gets read and translated by ribosomes into the amino acids that make up the dystrophin protein. The RNA gets read by ribosomes in groups of 3 RNA nucleotides (letters). These deletions of exons can cause the reading frame, which ribosomes are translating in 3 letter words, to be shifted. The subsequent RNA will encode for incorrect amino acids as well as lead to a premature stop codon that causes protein truncation & also nonsense-mediated decay of the RNA, and loss of dystrophin protein expression. The approach that Sarepta uses with eteplirsen, and BioMarin/Prosensa uses with drisapersen, is to induce skipping of another exon, in this case exon 51, to restore the proper reading frame.
There are lines of evidence in humans that this approach can restore functional dystrophin. One is that within a DMD patient's muscle, there are occasionally revertant fibers that have spontaneously skipped exons, and now have enhanced dystrophin expression in that fiber. An even stronger line of evidence that making this skipped-exon dystrophin protein can be functional comes from a related genetic disease called Becker Muscular Dystrophy (BMD). These patients have mutations in the dystrophin gene, but do not have loss of the reading frame, and are able to produce functional dystrophin. BMD patients sometimes have exactly the same mutation as DMD patients, except they are also missing an additional exon that maintains the reading frame. For instance, BMD patients can have deletion of both exons 50 & 51, whereas DMD patients can have deletion of just exon 50. What Sarepta and BioMarin are trying to do is turn the DMD patient into a BMD patient by skipping over exon 51 to produce the same dystrophin that is produced in exon 50-51 deletion BMD patients. BMD patients typically have a much milder clinical course than DMD patients, and BMD patients who have mutations that are equivalent to what would be induced by different exon-skipping approaches appear to have an even more favorable prognosis.
Exon Skipping Rationale (Wilton et al., 2015)
How Much Dystrophin is Needed?:
Proving that these therapies generate dystrophin is important to provide supporting evidence that the drug is doing what it is intended to do. Further, if a correlation can be made between the range of dystrophin that appears to lead to benefit in clinical trials, then future exon-skipping trials targeting patients with rare mutations could potentially use dystrophin as a surrogate endpoint for approval. There are still questions about how much dystrophin is necessary to lead to a clinical benefit, but emerging data from BMD patients suggest that potentially as low as 10% of normal dystrophin levels are sufficient to lead to milder clinical course - as quantified by Western blot. BMD patients below 10% had a more severe clinical course closer to DMD, although still potentially better than the typical DMD progression. In recent comments at the 2015 Morgan Stanley Healthcare conference, Sarepta CEO Ed Kaye indicated that the FDA will look at definitive production of dystrophin as supportive, but they aren't looking for a specific level of dystrophin production (discussion starts around 11:00 in MS webcast).
Sarepta's Dystrophin Data:
Immunohistochemistry - Percent Positive Dystrophin Fibers:
Method: Immunohistochemistry uses an antibody to visualize the expression of a given protein (dystrophin) in a tissue section. It is semi-quantitative and shows the localization of the protein, which for functional dystrophin should be at the muscle fiber's membrane. Counting the number of muscle fibers that appeared to be dystrophin-positive was the method used for the primary endpoint of the clinical trial, which was double blinded up to 24 weeks, at which time muscle biopsy tissue sections were scored & compared to baseline biopsies, to determine the increase in dystrophin-positive fibers. I do not believe there was a specific threshold for what constitutes a dystrophin-positive fiber, and was left to the interpretation of the evaluator, and thus this is potentially subjective, even if blinded. Speculating, but perhaps part of the reason this was used as the primary endpoint can be traced back to the origins of the concept of exon-skipping. Steve Wilton, one of the people who developed the exon-skipping approach in DMD, was one of the first to report endogenous exon-skipping that spontaneously occurs in DMD patients. Even back in 1997, he showed that these revertant (dystrophin-positive) fibers are induced by exon-skipping, and this is a potential therapeutic approach. Wilton has been involved in the development of eteplirsen, and perhaps this is part of the reason measurement of the number of dystrophin-positive fibers was used as the primary endpoint.
A description of the method was presented at the FDA dystrophin workshop, slides here.
Data:
My Take: They saw a consistent increase in dystrophin-positive fibers in patients at 24 weeks, but not at 12 weeks. Their conclusion was that it takes more than 12 weeks for enough dystrophin to accumulate due to the slow turnover of the protein. The placebo patients crossed over to receive eteplirsen at 24 weeks, and they also saw an increase in dystrophin-positive fibers at 24 weeks after starting treatment. The 30 mg/kg dose group saw a further increase in dystrophin positive fibers from 24 to 48 weeks, suggesting that dystrophin continues to accumulate. The amounts produced appeared similar between the 30 mg/kg group and the 50 mg/kg group. The 4th biopsy may add additional information about to what levels dystrophin is able to be maintained over long time periods of treatment (3+ years). All of the sections were scored by the same person, but the FDA seemed to want multiple people to score the sections & see the concordance rate. I expect this analysis to be part of the rescored biopsies that have been performed.
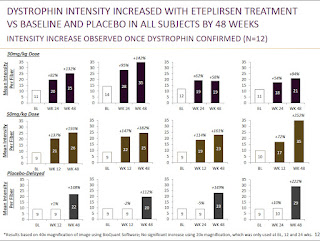
Immunofluorescence - Mean change in fluorescence intensity:
Method: They also performed analysis on the change in fluorescence intensity from baseline. There are a number of methods for measuring changes in fluorescence intensity that are similar and demonstrated to have good intralab & interlab concordance. The 3 methods used there are similar to each other, but I believe Sarepta (which did its analysis before the paper was published) did their analysis slightly differently than any of those 3 methods. Sarepta's method, to me, seemed most similar to the Taylor method, which creates a spectrin (muscle membrane protein) mask to only count presence of dystrophin at the membrane. Then the dystrophin intensity is taken for the whole image (not for each fiber individually). Sarepta did not appear to use a spectrin mask, but I believe said they used the trace dystrophin staining (or more intense staining in revertant fibers) that outlines the muscle fiber to determine the area to score dystrophin intensity from. In the Taylor paper (above) they found that this method had high concordance with the quantities determined by Western blot, but immunofluorescence was more sensitive at lower levels of dystrophin.
A description of the method was presented at the FDA dystrophin workshop, slides here.
Data:
My Take: While the dystrophin produced was distributed throughout many muscle fibers, as indicated by the increase in dystrophin-positive fibers to about 40% of normal, they only saw increases in total dystrophin intensity to about 15% of normal, which is more representative of the actual amount of dystrophin being made. This still is a significant change from baseline, as indicated by the individual patient data in the 2nd figure. This is also above the 10% threshold suggestive of benefit from the BMD patient data - although that number comes from data measured by the less sensitive Western blot assay.
The individual patient data shown in the bottom figure does raise some questions for me. For instance, they seem to be able to detect significant increases in dystrophin intensity at 12 weeks for some patients, and this wouldn't be as consistent with their dystrophin-positive fiber data that saw no increases at 12 weeks, which they attributed to the slow turnover of dystrophin. Additionally, there is a note at the bottom of the figure that this analysis is based on analysis of 40x magnification of the images, and that 20x magnifications were used at 0, 12, and 24 weeks, but no significant increases were observed. I don't know why one magnification would show increases, but the other wouldn't, and that would seem to be a concern. I would hope in the rescores or the 4th biopsy they follow exactly one of the three methods used in the IF quantification paper mentioned previously, which would give me further confidence in this data.
Western Blot:
Method: A Western blot is one of the most common ways of measuring a protein's presence. It is semi-quantitative, and has been used to measure the relative amounts of dystrophin in DMD vs. BMD vs. normal muscle biopsies. Sarepta has only so far presented Western blot data from a single patient. With a Western blot, the cells from a section of the muscle biopsy are lysed, and their protein contents are run on a gel that separates them by size, before being probed with an antibody to detect the presence, and roughly the quantity, of a given protein.
Data:
My Take: While there is clearly a band indicative of dystropin being produced, this Western is presented in a sloppy fashion. They clearly loaded much less total protein in the control lane from normal muscle, and yet even in the eteplirsen study paper they did not state how much total protein they loaded - my guess is a 10x or 15x dilution. The top row (presumably dystrophin) and bottom row (presumably loading control) aren't even labeled. Also the control sample is not adjacent to the 48 wks Tx sample, it has been placed there, but I assume all the samples were at least run on the same gel, otherwise they really cannot be compared. From this blot Lu et al., who reviewed data from the different exon-skipping trials, guessed that this was definitely not above the 10% threshold of normal dystrophin levels, but it's difficult to tell. If I had to guess, I'd say it's in the 5-10% range, but I expect them to have more thorough Western blot data tomorrow with a dilution series of control muscle (dilute control muscle sample down to 20%, 10%, 5% etc.) to get a better sense of the quantity. Lu et al. consider Western blots to be the preferred, but less sensitive, method of dystrophin detection compared to immunofluorescence - see figure below from Taylor et al. for concordance of WB to IF.
Concordance of Western Blot & Immunofluorescence
From Taylor et al., 2012
#1-5 DMD, 6-7 IMD, 8-12 BMD
One of the discordant points may be due to BMD mutation causing loss of membrane localization & thus low scoring based on immunofluorescence.
RT-PCR:
Method, Data & My Take: Another confirmatory measurement for dystrophin production by exon skipping is RT-PCR. RT-PCR can be used to look at the dystrophin RNA transcript and see if the drug is causing production of a smaller exon 51-skipped RNA. Sarepta has so far only shown data from 1 patient (different patient from the Western blot), but that patient appears to have an increase in the predicted exon 51-skipped RNA. I would like to see this analysis in all patients, if there is sufficient material from biopsies. This data, for me, is the least important measurement quantitatively, as long as they can show some band for the skipped product.
What Others are Saying About the Dystrophin Data:
I also would suggest taking a look at some of the reviews by those in the DMD field that have discussed the dystrophin & clinical trial data from Sarepta, BioMarin/Prosensa, and PTC Therapeutics (ataluren). These have all helped inform my opinion & most of the papers are freely available:
Lu et al.: What Can We Learn From Clinical Trials of Exon Skipping for DMD?
Hoffman & McNally: Exon-skipping therapy: a roadblock, detour, or bump in the road?
Merlini & Sabatelli: Improving clinical trial design for Duchenne muscular dystrophy
Wilton et al.: The emperor's new dystrophin: finding sense in the noise
Kole & Krieg: Exon skipping therapy for Duchenne muscular dystrophy
Conclusions:
The fact that the study was double-blinded at 24 weeks, and those analyzing the dystrophin data were blinded to the identity of the samples at the 48 week time point as well, gives me confidence that they are truly producing dystrophin above the level of detection in these assays. While there is expected to be variability from patient to patient in the amount of dystrophin produced, due to the fact that you are relying on a small biopsy to be representative of the entire muscle, the methods used should be able to detect if a potentially beneficial amount of dystrophin is being produced. The fact that they could measure it by a number of different assays gives me more confidence, and the amounts that they appear to be producing are in the area of what would be expected to be clinically beneficial (although this is still an open question). That is more evidence than can be said for other drugs intended to increase the production of dystrophin, such as drisapersen and ataluren, which have either not attempted or never showed very convincing dystrophin data. Dystrophin is the protein missing in DMD, and I feel it's essential to show production of dystrophin, both to demonstrate the drug is working as expected, as well as to make future clinical development easier. I listed a number of concerns with the different methodologies they used, and perhaps in the past the FDA may have had concerns as well, and I hope, and expect, to see cleaner data tomorrow.
The key things I'll be looking for tomorrow from the dystrophin data are:
Immunohistochemistry - percent-positive fibers: rescores by independent evaluators are similar to the original scores.
Immunofluorescence - intensity: the amounts at 4th biopsy, and did they use a different method for calculating the IF intensity.
Western blot: amount at 4th biopsy, data for more patients, and a dilution series of normal muscle as a control to better determine the amount of protein being produced in treated patients.
RT-PCR: data from more patients.
I wrote a follow-up post here with my thoughts on the updated data.
Disclosure: I own shares of Sarepta