There is one missing mechanism from the armament of anti-HBV therapies I have seen (see this recent review for comprehensive list of therapeutic strategies being developed). It is one targeting the most mysterious HBV protein of them all, the X protein. The X protein was named because its function was unknown at first, but after years of research, it has been elucidated that it is crucial in modulating the expression of HBV genes, and could have other functions in depressing inflammatory signaling. I cited a schematic of the various features and functions of the different domains of the X protein below, for those interested in studying the biology of the protein further, in particular the promoter transactivation functions.
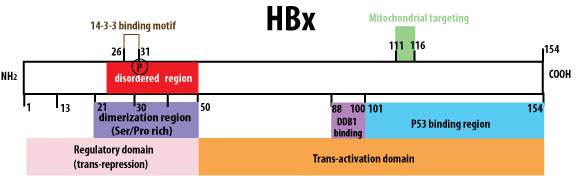 |
| Image from http://education.expasy.org/images/HBX_regions.jpg |
The role of the X protein as transcriptional transactivator is potentially very promising as a therapeutic target. It has been found that the X protein is absolutely crucial for HBV infection. A mutant HBV without the X protein completely fails to infect humanized mice, and a similar mutant woodchuck hepatitis virus fails to infect woodchucks. What is interesting is that later HBV X protein expression appears to be able to rescue these genomes and have productive HBV expression subsequently.
The objective of many HBV researchers right now is to remove covalently closed circular DNA, or cccDNA, the genome of Hepatitis B virus. An alternative to this same goal would be to epigenetically inactivate the cccDNA genome, thereby stoping productive virus production and essentially having dead genomes inside the mouse. HBV X protein appears to be necessary to keep an open and active chromatin state on cccDNA. Without it, the genome becomes silent explaining the "dead genomes" phenomenon. Patients with acute HBV infection and recovery can often still harbor cccDNA that is detectable by PCR for years after.
It is suggested here that investigators should pursue a small molecule screen that antagonizes X protein function. Such a molecule would be specific to HBV proteins, and turn off viral infection almost like a switch. The production of all viral proteins would be suppressed simultaneously, including the key serum biomarker HBV surface antigen, or HBsAg.
In order to design the small molecule screen, a few considerations need to be undertaken. HBV X helps to transactivate many different promoters, including the HIV LTR. Luciferase or GFP expressed by the LTR promoter could be assayed for expression levels influenced by X protein as a convenient readout for high throughput screens. A negative screen of cells without the X protein would need to be taken out as well to rule out compounds with non-specific function.
Lead candidates from this screen would need to be tested against the "real thing" and HBV itself. A common cell line previously used for this is the HepG2.2.15 cell line, which harbors an integrated HBV genome. However, it's not clear that the HBV X protein's function is the same for an integrated chromosomal HBV versus HBV in its natural state as a circular episome. The same question is true for many studies on the function of X protein using an HBV linear over-length genome contained on a bacterial plasmid.
In order to test the lead compounds against something closer to the "real thing," a model that has cccDNA must be used. A cell line HepG2-hNTCP, which expresses the HBV receptor human NTCP, has been recently described allowing HepG2 cells to become infected with HBV. The efficiency of infection in vitro remains very low however. For a high-throughput screen, it might be more advisable to have a more efficient process producing cccDNA inside cells. There are two potential ways of doing this. One would be to transfect cccDNA itself, which might be generated by a PCR and ligation process. The other would be to transfect purified HBV capsids, which pseudo-infect HepG2 efficiently. Whatever the method, one would want to test controls of genome without X protein and cells alone, in order to measure any possible side effects of the drug in liver cells.
The alternative strategy compared to a small molecule screen would be a rationale design of a small molecule using the known crystal structure of the DDB1 and HBV X binding surface. HBx binds to DDB1 with an alpha helical loop. If a small molecule could be designed that fits into this binding pocket, then an efficient anti-HBV molecule might be obtained with the efficacy of potential complete knockdown. Furthermore, many viruses co-opt DDB1 for their life cycles using this same interface, meaning that any discovered small molecule compound could leveraged against multiple indications, which should make companies and investors happy.
While I outlined novel small molecule means of targeting the X protein, in theory, some of the siRNA's being developed target the X protein transcript (see Arrowhead and Alnylam), and might have some mechanism in decreasing X protein levels. This might explain some of the differences in knockdown abilities among the different transcripts, since it has been shown that most siRNA's target transcripts around the core region are not very efficient at knockdown. Targeting surface antigen transcripts with siRNA's is also often less efficient than one's targeting all HBV transcripts. That said, the efficacy of siRNA's against the X mRNA might be limited since these other HBV mRNA's greatly outnumber the X transcript, and therefore sop up most of the siRNA efficacy against the X mRNA transcript.
In conclusion, directly targeting the HBV X protein is a tantalizing option for a new therapeutic strategy that is not currently being pursued by drug companies today, but may fit well with cocktail HBV therapy approaches, such as the one Arbutus (formerly Tekmira) is pursuing.