by Robert Kruse
Cellectis ($CLLS) has sparked much interest among investors, scientists, and patients for the allogeneic CAR T cell therapy platform. It offers the potential for a centralized manufacturing of T cells at a single site, and then distribution of frozen vials around the world to hospitals to infuse locally to cancer patients. The manufacturing, distribution, and payment model fits well within pharmaceutical paradigms today. This contrasts with the autologous approaches being pursued by Juno ($JUNO), Kite ($KITE), and Novartis ($NVS) for their CAR T cell therapy platforms.
The Cellectis strategy consists of a few key elements: (note I will focus on their lead UCART19 product and not their other proposed strategies. more information here).
1) Knockout of the TCR genes removes the ability of allogeneic cells to bind to host MHC class I molecules and become activated, attacking host tissue (graft versus host disease). Another human's T cells will not have undergone thymic deletion to your different set of HLA molecules, which is the biological reason behind this discrepancy.
2) Knockout of CD52 in transfused allogeneic cells allows the use of an anti-CD52 antibody (Alemtuzumab) to kill host T cells, providing an ongoing selective advantage for the transfused T cells, and also to prevent host T cells from attacking those T cells.
3) General chemotherapy and lymphodepletion, like the UPenn and Novartis protocols, helps CAR T cell homeostatic expansion, but also in the case of Cellectis, helps protects their cells from other immune effectors, which would recognize the varied MHC class I molecules on the infused product surface as foreign.
4) Suicide gene in addition to CAR T cell expression for additional safety feature to eliminate the T cells
5) Cellectis has an opportunity in the market for patients whose chemotherapy has rendered their cells ineffective donors. Having healthy T cells from another human being should be a major advantage in this instance.
There are potential issues with this approach that I have yet seen discussed among press releases by the company and other scientific commentary. Prominently, I see the potential for the Cellectis strategy to make the patient highly susceptible to infections, mitigating the ability of the therapy to achieve its endpoints.
Before the start of therapy, the patient is conditioned to remove most of their current immune cells. This lymphodepletion leads to loss of T and B cells fighting infection. CAR T cell therapies right now are similar to patients undergoing bone marrow transplants. When one infuses the allogeneic CAR T cells, these cells aren't matched to the patient's HLA molecules, so can't recognize any viral peptides inside host cells. Indeed, the CAR T cells don't even have TCR molecules even if Cellectis did try to match donor CAR T cells to patients' HLA types. During the provision of cancer therapy, the patient must constantly be in an immunosuppressed state in order to avoid Cellectis' CAR T cell destruction by the host immune system, which alternatively makes the patient highly susceptible to infections. The T cell population in the patient is mostly incapable of the regular job of surveying peptides inside cells, presenting major potential issue in the clinical management of these patients, who could perhaps require hospitalization during the duration of their CAR T cell treatment while immunosupressed, causing the costs of the procedure to skyrocket, in spite of the initial allogeneic manufacturing costs being much cheaper. I am making an assumption here that the UCART19 clearance of cancer will take 20-30 days, similar to the autologous trials from UPenn. In the UPenn trials, the most monitored time is just the first few days for cytokine release syndrome, but otherwise the patients can leave the hospital after emerging from this window and return as necessary.
Contrast this with the approach of Juno, Kite, and Novartis. The patient is lymphodepleted, but the infused autologous CAR T cells are polyclonal in their TCRs and should have the capacity to fight off any infections, in essence, re-constituting the immunity to pathogens in addition to their tumor fighting capabilities.
A solution that Cellectis or any other company could pursue is a compromise of the two models. The answer is possibly found with Bellicum's ($BLCM) strategy of infusion allogeneic haploidentical T cells in order to provide infection protection in bone marrow patients, and some measure of graft versus host disease. It could easily be imagined that these haploidentical cells retaining TCR genes could be manufactured with CAR T cells. A bank of CAR T cells could be created with various HLA types, and then infused into patients with some partial HLA matches in order to provide a measure of limited infection protection. Of course, these partially HLA-matched T cells would likely cause some measure of GVHD in some patients meaning that a safety switch would be needed (Cellectis has their own safety switch for this purpose). As an aside, this proposal is different from allogeneic (but not off-the shelf) CAR-T cells that are made from donor T cells after an allogeneic transplant, with some data recently presented at ASH here. Furthermore, lymphodepletion would still be necessary in the protocol to protect the proposed allogeneic T cells from being recognized as foreign. Nevertheless, this strategy of using partially HLA-matched allogeneic T cells as the CAR T cell effector would solve the infection problem and permit a safer and more judicious use of allogeneic products for cancer immunotherapy.
Welcome to Biotechr
Biotechr is written by Dr. Robert Kruse (@RobertLKruse), who holds a PhD and is currently completing his MD. His research work focused on infectious disease and immunology. This blog is focused on analyzing the latest developments in biotechnologies being developed in academia and industry, with a particular focus on biomedical therapeutics. I hope that the posts are interesting and useful, and hope you join in the discussion with guest posts on the site!
Disclaimer: The thoughts on this blog are not intended as any investment advice regarding any companies that might be discussed, and represent my opinion and not the opinions of my employer. This site is not designed to and does not provide medical advice, professional diagnosis, opinion, treatment or services to you or to any other individual.
Friday, December 11, 2015
Friday, December 4, 2015
Final Thoughts on Bluebird Prior to ASH
Here's what I'll be looking for in the ASH data bluebird bio will present over the next week. I thought it was worth a short post since there has been some new and relevant data relating to the BCMA CAR-T program partnered with Celgene, and also there was some confusion before/during their Piper Jaffray presentation over the path forward with LentiGlobin. I previously wrote a more detailed post on the data in the ASH abstracts for bluebird's LentiGlobin gene therapy product here.
LentiGlobin in B-Thalassemia
My main focus for B-Thalassemia will be how the new data influences the development path forward. Bluebird presented at the Piper Jaffray healthcare conference where they tried to clear up some confusion over whether the development path for LentiGlobin had changed. The confusion came from an investor meeting prior to the conference (link). Bluebird reiterated that its development path had not changed. They added that they are constantly trying to improve their products, and they look at minor changes that can be incorporated into the current program, as well as other changes that might be for "next-generation" products that would require a new IND.
Just as a refresher, in May, bluebird gave an update on their regulatory path forward in June after discussions with both EU & US health agencies (link).
For the EU, they believe they could apply for conditional approval based on their current HGB-204 & 205 studies, which are expected to complete enrollment soon. Importantly, the proposed primary endpoint would be transfusion reduction. For the US, they believe they could apply for accelerated approval based on their pivotal studies HGB-207 and 208, which have yet to be initiated (15 B-Thal patients per study), with accelerated converting to full approval based on longer term follow-up data. For the US, the proposed primary endpoint is transfusion independence. While this was the current thinking in May, the plans were subject to change with further data and discussions with regulators.
Considering the consistent ability, so far, of LentiGlobin to induce transfusion independence in non-B0/B0 B-Thalassemia patients, but not B0/B0 patients, bluebird has mentioned the possibility of developing the product differently for these 2 populations. However, bluebird emphasized it was still too early to be making decisions yet. To completely speculate with this in mind, and the above regulatory outlook, I could see bluebird developing LentiGlobin, as is, for the EU since transfusion reduction is the proposed primary endpoint, and LentiGlobin should cause reduced transfusions in B0/B0 patients as well. For the US, I could see them splitting off the B0/B0 patients, and focusing on the non-B0/B0 patients, which they say are 2/3rds of the addressable population. If, later, they can improve the amounts of T87Q hemoglobin LentiGlobin treated patients produce to consistently 8+ g/dl with either future production tweaks or follow-on products, then I could see them going after the B0/B0 genotype again, with the goal of consistent transfusion independence.
So with this in mind, what I'll be looking for in the B-Thalassemia data from their ASH data is - are 100% of the non-B0/B0 patients transfusion independent (or trending) by 1 year post-treatment, and what is the level of transfusion reduction in B0/B0 patients?
LentiGlobin in Sickle Cell Patients
There has not been as much recent discussion about LentiGlobin for sickle cell disease (SCD), but I'll be looking for longer follow-up on the 1st treated patient on clinical & blood endpoints, and their current T87Q level. I would not expect much of an increase in T87Q at this point, as I've mentioned in previous posts, however, the ~50% T87Q would seem likely to be sufficient for significant clinical benefit.
There will also be a poster presentation with data on at least 2 new patients treated with LentiGlobin. These patients were treated with a lower cell dose than the first patient, and also one patient had a lower vector copy number (VCN) in the drug product. So I will be looking for the levels of T87Q these patients have been able to produce, both in terms of the total amount of T87Q and the relative amount of T87Q to sickle hemoglobin (HbS).
Bluebird has also suggested that they have been performing assays to determine the percent of modified cells, in addition to just giving the average VCN across all cells. This is another way to measure how well they are able to transduce the CD34+ cells with the LentiGlobin vector in their drug product, and potentially track the levels in the patient. I could see this being a useful number to know, particularly for SCD, where in hematopoietic stem cell transplants (HSCT), mixed chimerism can occur, with data suggesting that you only need 10-30% of HSCs to be corrected to functionally cure a patient. I previously wrote about mixed chimerism and other considerations for LentiGlobin in SCD in more detail with Zack, @BioTerp, which can be found here. How much a single copy of the LentiGlobin vector can protect a red blood cell from sickling is unknown, but is likely to be at least somewhat beneficial based on levels of fetal hemoglobin of 30% being known to be protective.
BCMA CAR in Multiple Myeloma
The importance of ASH data for Bluebird's BCMA CAR program has increased, as an additional late-breaking abstract will have 1st in human clinical data of a different BCMA CAR in a phase I trial at the NIH - to be presented on Tuesday December 8th.
Bluebird has mentioned the study in recent webcasts, and I expect them to try to use it to draw excitement to their program. Bluebird itself has 3 poster presentations at ASH on their BCMA program (1, 2, 3), however, I would say none of them really adds that much new data to what they've already presented. On using CD28 versus 4-1BB as a costimulatory domain, it is still too early to tell the differences in safety or efficacy yet for CD19 targeted CARs. A recent preclinical paper suggests that on a per cell basis, CD28 costimulatory cells have greater tumoricidal activity whereas 4-1BB costimulatory cells have better persistence. In the clinic, results using either costimulatory domain have been impressive, but it's still too early to tell if either has an advantage - reviewed here. Despite the potential differences in the CAR construct, my confidence and interest in bluebird's BCMA program has increased after the release of this new abstract, and I will be very interested in any updates from the NIH trial.
Competing Approaches in B-Thalassemia and SCD
Lastly, a big thing I will be keeping an eye on at ASH are data from other approaches in B-Thalassemia and SCD. Specifically, I am interested in clinical data from Bellicum on their approach to make allogeneic HSCTs safer (including in B-Thalassemia and SCD), clinical data from Global Blood Therapeutics on their small molecule drug for SCD, and preclinical data on a different gene therapy approach for both diseases by Sangamo. I discussed them a bit more in my previous post on ASH abstracts. Additionally, Timothy Sullivan had a very good post on Bellicum's approach here. One interesting way bluebird could improve the safety of their approach compared to even the presumably improved-safety allogeneic approach of Bellicum would be to change their conditioning regimen, such as depleting only CD34+ cells, instead of myeloablation with busulfan. This might improve some of the negative effects of the conditioning regimen, such as immune recovery. Bluebird has mentioned this as a possibility, so it will be interesting to follow any potential updates on that, however, this would be a significant change and would certainly be a follow-on product requiring separate clinical development.
Edit: @BioTerp also reminded me about additional similar approaches using viral transduction of HSCs with a hemoglobin (or modified hemoglobin) expressing vector - one being developed by Donald Kohn, whose lab has published many preclinical papers here, as well as another from MSKCC with economic partner Errant Gene Therapeutics. Although I do not believe there will be new data from either group presented at ASH.
Conclusion
There will be a lot of new information coming out of ASH to consider for bluebird - new LentiGlobin data, 1st in human BCMA CAR data, as well as early data from other competing approaches. I'll be looking for more clarity on the development pathway for LentiGlobin, and the consistency of the results given the current treatment protocol. I think the BCMA CAR data from the NIH program might generate additional focus on bluebird's program, and it will be interesting to see how bluebird tries to frame that data in the context of their program.
Disclosure: I own shares of BLUE, BLCM, SGMO
Thursday, November 12, 2015
Bluebird Bio's Lentiglobin - Is the Sky Falling? (ASH 2015 Abstract Review)
While I don't think the sky is falling for bluebird's LentiGlobin, sentiment is certainly changing, as the story "seems" to have shifted from "it can cure every patient" to "the therapy wears off over time and it doesn't work for everybody". However, I feel this is more a shift in sentiment than their data getting significantly weaker. I think people were way too optimistic if they were expecting cures for every patient, but the pendulum may have swung a bit too far to the negative side. So here I'll take a look at bluebird bio's most recently released data in their 2015 ASH abstracts and how the whole picture is coming together for the efficacy of LentiGlobin.
I've recently written a number of other posts on bluebird, mostly on LentiGlobin in sickle cell disease (SCD) and B-Thalassemia and their results presented at the EHA meeting in June. Check them out if you want additional context for this post:
Posts 1, 2, 3, 4.
The abstract that appears to be the main focus of people's attention is on further follow-up data on patients in the Northstar (HGB-204) study, which is enrolling patients with B-Thalassemia.
So they've treated 10 patients with B-Thalassemia major by July 31st with LentiGlobin, 5 with the B0/B0 genotype, where no functional HbA hemoglobin is made, and 5 who are heterozygous for B0 and have one functional or hypomorphic copy of beta globin (BE etc.). The headline is that none of the 3 patients evaluable (>6 months follow up) who have B0/B0 became transfusion independent. However, the 4 evaluable patients with non-B0/B0 all have been transfusion free for >90 days. Up until now all patients treated (only 4 - 2 in the Northstar study, 2 in HGB-205) had achieved transfusion independence for at least 90 days of follow up. While perhaps some had expectations that every patient would achieve transfusion independence, that was always unlikely to be the case based on their previous data. Total hemoglobin needs to be at least >8 g/dl (probably better if 9-10 g/dl) to avoid transfusions, and looking back at their data from last ASH shows this might be right at the edge of what they're able to achieve with LentiGlobin for B0/B0 patients. Here's the data on their first 4 patients they presented last year:
3 patients had B0/BE (patients 1201, 1202 and 1102) and reached transfusion independence. You can see those patients are still getting a contribution in their total hemoglobin from HbE (from BE allele) and HbF (fetal hemoglobin). The B0/B0 patient, 1106, whom they also called transfusion free, only has HbF to add to their HbA-T87Q hemoglobin. While they were off to a good start with already 6.8 g/dl T87Q being made, it would probably need to get ~8 g/dl to achieve stable transfusion independence, assuming they continue to get some contribution from HbF.
It appears that this patient was one of the two B0/B0 HGB-204 patients that required a single transfusion, based on the new ASH abstract. I haven't heard many discussing this B0/B0 patient, and it would have potentially made for an even worse headline of having a previously "cured" patient no longer being cured. This was basically the headline a couple weeks ago when a patient treated with an older version of the T87Q expressing vector required a transfusion after 7 years of being transfusion free. I'll get back to my thoughts on that patient later.
Getting back to the more recent B0/B0 patient, I had hoped that her T87Q hemoglobin would increase enough to remain transfusion independent. You can see they still have some HbA (blue) from previous blood transfusions in their total hemoglobin, and this would need to be made up for by the patient's own hemoglobin after the transfused cells have completely gone. So it was probably premature to announce this patient as "transfusion free" (ASH 2014 PR), and it's probably best to wait until all the transfused HbA goes away. In any case, having a single transfusion over the course of about a year is still a significant reduction in transfusions considering the entry criteria for the study requires at baseline at least 8 transfusions per year or >100 mL/kg/year of transfusions. It's also possible the patient will become transfusion independent if their T87Q production goes up a bit more, which it has in some of their previously treated patients. So, I will be interested if their production of T87Q has increased, and by how much, since last year. Importantly, this patient had a relatively high vector copy number (VCN) in their CD34+ LentiGlobin product (1.5), so if they wanted to more consistently achieve >8 g/dl T87Q in B0/B0 patients they might need to achieve ~2 VCN, like in patient 1202, who has now been holding steady at around 9.7 g/dl T87Q for almost a year.
How tight a correlation between VCN, CD34+ cell dose, and the quantity of T87Q patients produce will become clearer as they continue to treat more patients. My general feeling is VCN will be more important for determining peak levels of T87Q. However, I could imagine low CD34+ cell doses might cause reduced chimerism - where a lower percentage of old hematopoietic stem cells get replaced by transplanted ones - and thus cause lower levels of T87Q production. So far, though, it seems like the VCN they see in the LentiGlobin product they administer is similar to the amount they see in patients' peripheral blood, so they are likely getting relatively good reconstitution:
Another thing I noted from the abstract was that a patient had relatively modest T87Q production (just 1.9 g/dl), as well as one patient having very low VCN in the CD34+ cell product they received (just 0.3 VCN). It will be interesting to see if the patient given the 0.3 VCN product is also the one producing the very low levels of T87Q, and if this is the B0/B0 patient who is still considered transfusion dependent. It's surprising a patient was treated with that low VCN, as I thought the original release criteria for LentiGlobin on this study was 0.5 VCN - page 43 here.
Turning, briefly, to the other 2 abstracts, which had less significant data updates, the abstract for the HGB-205 study includes the first sickle cell patient treated (previously reported data at this year's EHA meeting), and follow up data on patients 1201 & 1202, whom I mentioned above in the 2014 ASH data.
All this data looks consistent with previous releases, both B-Thalassemia patients have maintained their T87Q production steady for over a year. The SCD patient's T87Q levels continued to increase now at 9 months compared to 6 month data at the EHA meeting. In SCD, the ratio of sickling hemoglobin (HbS) to anti-sickling hemoglobin (T87Q + HbF) is more important than the absolute quantity (g/dl) of T87Q produced. The levels bluebird believes will be sufficient to significantly reduce symptoms is 30% anti-sickling hemoglobin, which they have already achieved. I am a bit more conservative, as I had outlined in previous posts, about what percentage one might need to achieve, thinking it could be slightly higher than 30% depending on how evenly the T87Q hemoglobin is distributed throughout the patient's red blood cells. However, the ~50% anti-sickling hemoglobin achieved in this patient is impressive, and I would expect to be therapeutically beneficial. So far the patient does not have any reported no hospitalizations or blood transfusions. It is not surprising that the T87Q levels are leveling off somewhat. They're actually slightly higher than I would have expected at 48% now from 40% at 6 months. A major reason for the large increases in the %T87Q in the patient's blood from months 3-6 came from having their previously transfused blood cells (carrying HbA) die off, raising the relative amount of T87Q. So I am not expecting a significant increase in T87Q% when they present updated data at ASH. I will be looking to see the maintenance of T87Q levels, and if the other efficacy measures in this patient continue to look promising.
Lastly, the abstract for the HGB-206 trial contains only information on the LentiGlobin drug product that was infused into 2 patients with SCD.
It's dangerous to mobilize hematopoietic stem & progenitor cells in SCD patients to enter the blood stream, which is how CD34+ cells were collected for the previously treated B-Thalassemia patients. So SCD patients need to have CD34+ cells harvested directly from bone marrow. This typically leads to lower amounts of CD34+ cells, as you can see the cell dose in these two patients is <3 x10^6 cells/kg, compared to 5.6 x10^6 cells/kg for the previous SCD patient, and normally 8 x10^6 cells/kg or higher in their previously treated B-Thalassemia patients. The first patient listed in this abstract also has a fairly low VCN of 0.5/0.6. It will be important to see how this low of a CD34+ cell dose affects the kinetics and total production of T87Q in these patients. For the patient with the 0.5/0.6 VCN dose (compared to 1.2/1.0 VCN for the SCD patient we have 9 month data on) they might produce significantly less T87Q. This patient may not make the 30% threshold bluebird has been using, however, an increase to 10-20% of anti-sickling hemoglobin could still have some therapeutic benefit.
Transfusions needed patient treated with original HPV569 vector
Another recent event was the disclosure that Subject 3, a B0/BE patient who had achieved transfusion independence after being treated with a previous version of bluebird's vector, required 2 transfusions after almost 7 years of transfusion independence. Since people were hoping for sustained transfusion independence, this news certainly sounded disappointing with potential implications for the duration of the efficacy for this approach. However, if we look at the levels of T87Q and total hemoglobin this patient had, they were always on the borderline of needing a transfusion, making only 2.7 g/dl T87Q and having total hemoglobin levels fluctuating between 8 and 9 g/dl over the past 5 years.
Importantly, bluebird noted in their press release that the levels of T87Q and VCN levels in peripheral blood have remained "largely unchanged". Unfortunately, the data have not been released to see exactly what "largely unchanged" means, but I doubt there was a sudden drop in T87Q based on the modest but relatively stable levels of T87Q the patient was producing. It does warrant keeping an eye on to see if T87Q levels do drop over long time periods. If there had been silencing of the viral construct, or perhaps an immune reaction against cells containing the transgene, one would have imagined there would have been a preferential drop in T87Q compared to total hemoglobin levels, which does not seem to be the case here. It's unclear how long viral gene expression can last in cells, but there is some data for transduced T cells that it can continue to last over 10 years, and potentially longer. 1st generation retrovirally transduced CAR-T cells still can be found with CAR expression more than a decade later. Additionally, there is another presentation at ASH where the authors retrovirally transduced a Thymidine Kinase safety switch into transplanted T cells, and they also saw that expression can be maintained for over 10 years. However, it will take further follow up in more patients before we get a better sense of the duration of efficacy for bluebird's LentiGlobin.
Sangamo also brought up in their 3rd quarter conference call concerns about the lentiviral integrations in this patient, and clonal dominance. Previously, cells containing a viral integration near HMGA2 grew in prevalence, a potential concern suggesting preferential growth of this clone. However, that clone ended up decreasing over time:
Other clones have increased in prevalence among the vector-modified cells recently, but bluebird suggests this is potentially due to random increases and decreases in prevalence over time, which could be exacerbated by the fact that so few cells were efficiently transduced such that, inherently, any given clone could grow or shrink considerably. There were only approximately 100 integration sites for the original patient treated, whereas there were many more in the more recently treated patients. An integration site analysis of their more recent patients suggests they are currently maintaining a very polyclonal population of engrafted cells:
Additionally, other self-inactivating (SIN) lentiviral vectors similar to bluebird's are also showing improved safety compared to earlier gamma-retroviruses so far, as well as signs of efficacy. A number of them are being presented at the same ASH session as bluebird:
- Safety and Clinical Benefit of Lentiviral Hematopoietic Stem Cell Gene Therapy for Wiskott-Aldrich Syndrome
- Gene Therapy Using a Self-Inactivating Lentiviral Vector Improves Clinical and Laboratory Manifestations of Wiskott-Aldrich Syndrome
Safety & efficacy data in Wiskott-Aldrich Syndrome was also published here with integration site analysis.
Continued safety, in particular the risk of insertional oncogenesis, is still a significant consideration, but I think the data so far suggests these SIN-lentiviral vectors are likely considerably safer than earlier gamma retroviral ones.
Conclusions:
In conclusion, I don't think the data presented here is out of line with bluebird's previously released data, but expectations were probably too high. LentiGlobin, with the average VCN and CD34+ cell dose given so far seems to be able to consistently induce transfusion independence in B-Thalassemia patients with B0/BE. In B0/B0 patients, the average VCN and cell dose do not seem to consistently be able to produce sufficient levels of T87Q to achieve transfusion independence, but will still likely be therapeutically beneficial and cause transfusion reductions. If bluebird can more consistently treat B0/B0 patients with VCNs on the higher side of what they've been able to achieve so far, it's possible they could achieve transfusion independence. It's also possible that currently treated patients will have T87Q levels increase over time to those sufficient for transfusion independence, but I'm not expecting large increases 6 months post-treatment. I think we will continue to learn considerably more about the relationship between VCN, CD34+ cell dose, and the kinetics of T87Q production from their upcoming presentations at ASH and in subsequent data updates. Bluebird has stated that only about 1/3rd of B-Thalassemia major patients are of the B0/B0 genotype, and their therapy will still probably be beneficial to those patients by reducing transfusion frequency, if not transfusion independence. Their data in the first SCD patient looks very promising, but the follow-up is still early, and subsequent SCD patients may have been treated with less efficacious doses. In total, I still think bluebird's approach appears to be of significant therapeutic benefit if the results they've seen so far continue.
None of my blog posts are intended as investment advice, and I intentionally don't typically go into valuation, I normally focus only on my interpretation of the data. There are, however, additional approaches for treatments in B-Thalassemia and SCD that are being presented at ASH that look promising as well. So I'll briefly look at a couple below:
Additional approaches in B-Thalassemia and SCD being presented at ASH
There are a number of other companies with interesting therapeutic approaches to B-Thalassemia or SCD that will be presenting pre-clinical and clinical data at ASH as well, they include:
Bellicum - Allogeneic HSCT is curative in patients with B-Thalassemia and SCD, in addition to other monogenic blood diseases and also is potentially curative in the treatment of hematologic malignancies. Bellicum is trying to make allogeneic transplants safer by adding back T cells containing their inducible caspase safety switch to T cell-depleted allogeneic transplants. Adding back T cells potentially can improve early immune reconstitution and hopefully reduce potentially lethal infections. Their safety switch is added to improve the safety of having donor T cells in the transplant, so T cells can be depleted if they begin to cause GVHD. Bellicum will be presenting at ASH on this approach, including in patients with B-Thalassemia. They have stated already that 4 patients with B0/B0 Thalassemia have been treated without complications and are transfusion independent. It's not surprising the transplant is curative, but the main things I will be looking for are levels of infection (and maybe measures of virus-specific T cells), as well as instances of GVHD and how well it's handled by activation of the suicide switch. In a number of patients from earlier studies, the approach has worked quite well in resolving GVHD rapidly, while surprisingly maintaining antiviral immunity post-depletion, published here.
For me, I have not researched enough to know for how many patients this would shift the risk:reward balance in favor of doing HSCTs, but I think how Bellicum's approach might effect this balance will become clearer as their data matures.They have 3 ASH abstracts on this approach:
- BPX-501 Cells (donor T cells transduced with iC9 suicide gene) Are Able to Clear Life-Threatening Viral Infections in Children with Primary Immune Deficiencies Given Alpha/Beta T-Cell Depleted HLA-Haploidentical Hematopoietic Stem Cell Transplantation (haplo-HSCT)
- Clinical Outcome after Adoptive Infusion of BPX-501 Cells (donor T cells transduced with iC9 suicide gene) in Children Given Alpha/Beta T-Cell Depleted HLA-Haploidentical Hematopoietic Stem Cell Transplantation (haplo-HSCT): Preliminary Results of a Phase I-II Trial
- Immune Reconstitution after Adoptive Infusion of BPX501 Cells (donor T cells transduced with iC9 suicide gene) in Children Given Alpha/Beta T-Cell Depleted HLA-Haploidentical Hematopoietic Stem Cell Transplantation (haplo-HSCT): Preliminary Phenotypic and Functional Results of a Phase I-II Trial
Sangamo - Sangamo is targeting an erythroid-specific enhancer that controls BCL11A expression. BCL11A restricts fetal hemoglobin levels, and mutations that affect BCL11A, or an erythroid-specific enhancer, have been shown to cause higher levels of fetal hemoglobin in patients. Sangamo is using their zinc finger approach to try to disrupt one or both copies of the enhancer that controls BCL11A erythroid expression. BCL11A is not just expressed in erythroid cells, so targeting the erythroid-specific enhancer is probably a better therapeutic approach. This approach looks promising as an alternative way to induce therapeutically beneficial levels of fetal hemoglobin in gene-modified autologous transplants. They will be presenting a number of preclinical studies on the approach in oral presentations at ASH:
- Clinical-Scale Genome Editing of the Human BCL11A Erythroid Enhancer for Treatment of the Hemoglobinopathies
- Genome Editing of the Bcl11A Erythroid Specific Enhancer in Bone Marrow Derived Hematopoietic Stem and Progenitor Cells for the Treatment of Sickle Cell Disease
Some background papers on BCL11A and fetal hemoglobin:
2008: Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A.
2011: A functional element necessary for fetal hemoglobin silencing.
2011: Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing.
2013: An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level.
Global Blood Therapeutics - will also be presenting data at ASH on their oral small molecule drug for SCD.
GBT440, a Potent Anti-Sickling Hemoglobin Modifier Reduces Hemolysis, Improves Anemia and Nearly Eliminates Sickle Cells in Peripheral Blood of Patients with Sickle Cell Disease
Disclosure: I own shares of Bluebird and Bellicum, no position currently in Sangamo or Global Blood Therapeutics.
Friday, October 2, 2015
Thoughts on Sarepta's Final Dystrophin Data
Sarepta released updated data for both dystrophin measurements and clinical readouts for their 12 patient phase I/II trial yesterday prior to presenting at the World Muscle Society Conference. Before they released this new data, I wrote a post on their previously released data on dystrophin and what I was hoping to see in this new data. The clinical data Sarepta released will also be very important in the evaluation of the potential benefit of eterplirsen, but, at least for this post and the previous one, I wanted to focus on the dystrophin data. Here is the new data and my thoughts on it.
Link to their webcast of data presentation.
Immunohistochemistry - Percent Positive Dystrophin Fibers:
4th Biopsy
Sarepta presented data from 4th biopsies taken at 180 weeks after beginning treatment and performed on 11 of the original 12 patients. These were scored for their original primary endpoint, percent dystrophin-positive fibers.
In previous data at 48 weeks, they saw all 12 patients show a statistically significant increase in dystrophin-positive fibers, but 1 of the 11 patients here did not show an increase. Steve Wilton (at 29:45 in the webcast), in reference to the Western blot data I'll get to below, suggested an explanation for why 1 or 2 patients did not have measurable dystrophin production at this time point. He thought the data was still impressive, but that there are limitations to using a small biopsy to try to represent the whole muscle, such as variability in quality of the biopsy. He also said the low detection limit of the assays (particularly Western blot), and perhaps the different underlying genetic mutations, which are not all identical for the treated patients, could play a role. In terms of my expectations, I was hoping to see continued evidence of dystrophin production across all patients, as they had seen in their earlier data, but the limitations of biopsy are reasonable, as there is local variability in muscle condition.
As opposed to their initial set of 3 biopsies, for the 4th biopsy, Sarepta added 3 additional independent pathologists to score the sections. Having just one pathologist scoring all the sections appeared to be one of the concerns the FDA had over their original data.
As stated on the webcast, all the pathologists agreed on whether a given patient sample showed an increase in dystrophin-positive fibers. The pathologist from Nationwide (presumably the same who scored the original 3 biopsies) consistently scored samples as having higher percentages of dystrophin-positive fibers than the other three. Ed Kaye on the call suggested that due to that pathologist's extensive experience, and he/she was likely to be more sensitive to even faintly dystrophin-positive fibers. While I'm not sure if that's really that satisfactory of an explanation, I don't think it is all that important. While the 3 independent pathologists scored the treated samples as having fewer dystrophin-positive fibers, they also scored the untreated samples as having fewer, and actually the magnitude of the fold change from untreated to treated was higher for the 3 independent pathologists versus the one from Nationwide. Most likely the ones from Flagship were scoring fewer trace fibers that were only weakly dystrophin-positive. In general, I think this data is stronger for having the 3 independent pathologists score it, even if they scored the patient samples somewhat lower.
Rescore of 12 & 24 week biopsies
They also rescored the 12 & 24 week biopsies by their 3 independent pathologists using the same method.
Again, the independent pathologists scored the biopsies in the same direction as the original pathologist, but with a somewhat lower magnitude of change. The consistency of the ability to detect an increase in dystrophin-positive fibers across all the pathologists does give me more confidence in this assay.
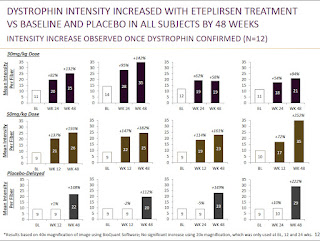
Immunofluorescence - Mean change in fluorescence intensity:
In addition to percent positive fibers, they also measured the fourth biopsy for change in the mean fluorescence intensity.
These results are very similar to those they already saw in the previous biopsies. While it's good to see that these results were still consistent at 180 weeks, I was hoping they would also do it with one of the other methodologies used for measuring fluorescence intensity, as mentioned in my previous post.
Western Blot:
4th biopsy
On the call there seemed to be a lot of focus on the Western blot data, and this is perhaps the assay the FDA most wanted to see for confirmation of the ability eteplirsen to produce detectable levels of dystrophin. 9 out of the 11 biopsies showed a detectable dystrophin band compared to 1 out of 9 patients with detectable dystrophin in an untreated exon 51-amenable comparator group. An example western blot is shown here:
It would appear they performed a standard curve, doing a dilution series of normal muscle tissue in lanes 2-4+, which I was hoping to see. Unfortunately, they did not label this at all, so I have no idea if it is a standard curve, and if so, how much normal muscle protein lysate was loaded in each lane. I was hoping they would present this information so we would be able to get a sense of what percent of normal dystrophin protein they were able to induce. They do say they loaded 50 ug total protein in each lane, which Steve Wilton on the call said was very conservative compared to what others have sometimes used to try to detect dystrophin in DMD patients by Western blot.
I was glad they showed the whole gel, and while it's not a beautiful Western, and the antibody has potentially non-specific bands underneath, it looks clean enough at the expected size for full length dystrophin.
In terms of the 9 of 11 showing a band, again, I was hoping to see a band for all treated patients, but this is probably the least sensitive assay of all of them. I'm not sure if the 2 of 11 not showing a dystrophin band here also had the lowest scores on the IHC/IF assays. They did have 1 of 9 from an untreated group of patients with a detectable band, presumably due to some level of endogenous exon skipping in that biopsy, but there does appear to be a clear increase in treated patients for detectable dystrophin by western blot.
Compared to my expectations, I was slightly disappointed with the Western data, with the understanding that there is expected variability in biopsies. I was also disappointed they did not label the standard curve in the presentation, so we would be able to get a sense of the amounts of dystrophin produced. However, from hearing Ed Kaye talk on this call, and previously, it seems the FDA is looking more for having convincing evidence of production of dystrophin than a specific amount. From that standpoint, compared to their original Western data, the new data does probably bolster the argument that they are truly producing detectable levels of dystrophin in a majority, if not all, patients.
RT-PCR:
They mentioned that they performed RT-PCR on all the muscle biopsies and were able to see the expected exon 51-skipped band. This indicates presence of the RNA that they were hoping to induce. They also sequenced the RNA to confirm the exon 51 skip at the nucleotide level. This is probably the most sensitive assay of the above for even very low levels of the skipped RNA transcript. For RT-PCR I was just hoping to see them perform the assay in all patients, and they did that.
Conclusions:
Before releasing this new data I was reasonably confident they were able to produce detectable levels of dystrophin, but with a number of questions over their methodology. While the updated data didn't address all my concerns, it seems like Sarepta tried to address the major concerns the FDA had, which appeared to be independent review by other pathologists for the immunofluorescence data, and Western blot data on more of the patients. I think the data are mostly positive in terms of validating the assays used, and that they are able to produce detectable dystrophin for long periods of time in a majority of patients. While that is probably the most important information for the FDA, my own optimistic expectations for the data were still slightly higher than what was presented.
Disclosure: I own shares of Sarepta
Wednesday, September 30, 2015
A look at Sarepta's dystrophin data pre-WMS15
Tomorrow, Sarepta will be presenting updated data on the ability of their exon-skipping drug eteplirsen to produce dystrophin in 12 Duchenne Muscular Dystrophy (DMD) patients that have been treated in a phase I/II study now out over 3 years. They have rescored dystrophin data from previous muscle biopsies, and they have also conducted a 4th biopsy in a number of the patients that have been analyzed for dystrophin production. They also are expected to present updated 6 minute walk distance as well as other functional data. The interpretation of both 6 minute walk data & dystrophin data have been controversial so far, but here I will focus on the data Sarepta has reported so far on the ability of their drug to produce dystrophin - the protein missing in patients with DMD. First, some of the basics that those familiar with the approach can skip over.
The Basics: Dystrophin & Exon Skipping
Dystrophin is a protein important for muscle fiber function & stability, and is the causal gene mutated in DMD. Many patients have deletions (or duplications) of segments of the gene, called exons, the parts of the gene that encode the segments of RNA that get stitched together (splicing) to make the final RNA product. The RNA gets read and translated by ribosomes into the amino acids that make up the dystrophin protein. The RNA gets read by ribosomes in groups of 3 RNA nucleotides (letters). These deletions of exons can cause the reading frame, which ribosomes are translating in 3 letter words, to be shifted. The subsequent RNA will encode for incorrect amino acids as well as lead to a premature stop codon that causes protein truncation & also nonsense-mediated decay of the RNA, and loss of dystrophin protein expression. The approach that Sarepta uses with eteplirsen, and BioMarin/Prosensa uses with drisapersen, is to induce skipping of another exon, in this case exon 51, to restore the proper reading frame.
There are lines of evidence in humans that this approach can restore functional dystrophin. One is that within a DMD patient's muscle, there are occasionally revertant fibers that have spontaneously skipped exons, and now have enhanced dystrophin expression in that fiber. An even stronger line of evidence that making this skipped-exon dystrophin protein can be functional comes from a related genetic disease called Becker Muscular Dystrophy (BMD). These patients have mutations in the dystrophin gene, but do not have loss of the reading frame, and are able to produce functional dystrophin. BMD patients sometimes have exactly the same mutation as DMD patients, except they are also missing an additional exon that maintains the reading frame. For instance, BMD patients can have deletion of both exons 50 & 51, whereas DMD patients can have deletion of just exon 50. What Sarepta and BioMarin are trying to do is turn the DMD patient into a BMD patient by skipping over exon 51 to produce the same dystrophin that is produced in exon 50-51 deletion BMD patients. BMD patients typically have a much milder clinical course than DMD patients, and BMD patients who have mutations that are equivalent to what would be induced by different exon-skipping approaches appear to have an even more favorable prognosis.
Exon Skipping Rationale (Wilton et al., 2015)
How Much Dystrophin is Needed?:
Proving that these therapies generate dystrophin is important to provide supporting evidence that the drug is doing what it is intended to do. Further, if a correlation can be made between the range of dystrophin that appears to lead to benefit in clinical trials, then future exon-skipping trials targeting patients with rare mutations could potentially use dystrophin as a surrogate endpoint for approval. There are still questions about how much dystrophin is necessary to lead to a clinical benefit, but emerging data from BMD patients suggest that potentially as low as 10% of normal dystrophin levels are sufficient to lead to milder clinical course - as quantified by Western blot. BMD patients below 10% had a more severe clinical course closer to DMD, although still potentially better than the typical DMD progression. In recent comments at the 2015 Morgan Stanley Healthcare conference, Sarepta CEO Ed Kaye indicated that the FDA will look at definitive production of dystrophin as supportive, but they aren't looking for a specific level of dystrophin production (discussion starts around 11:00 in MS webcast).
Sarepta's Dystrophin Data:
Immunohistochemistry - Percent Positive Dystrophin Fibers:
Method: Immunohistochemistry uses an antibody to visualize the expression of a given protein (dystrophin) in a tissue section. It is semi-quantitative and shows the localization of the protein, which for functional dystrophin should be at the muscle fiber's membrane. Counting the number of muscle fibers that appeared to be dystrophin-positive was the method used for the primary endpoint of the clinical trial, which was double blinded up to 24 weeks, at which time muscle biopsy tissue sections were scored & compared to baseline biopsies, to determine the increase in dystrophin-positive fibers. I do not believe there was a specific threshold for what constitutes a dystrophin-positive fiber, and was left to the interpretation of the evaluator, and thus this is potentially subjective, even if blinded. Speculating, but perhaps part of the reason this was used as the primary endpoint can be traced back to the origins of the concept of exon-skipping. Steve Wilton, one of the people who developed the exon-skipping approach in DMD, was one of the first to report endogenous exon-skipping that spontaneously occurs in DMD patients. Even back in 1997, he showed that these revertant (dystrophin-positive) fibers are induced by exon-skipping, and this is a potential therapeutic approach. Wilton has been involved in the development of eteplirsen, and perhaps this is part of the reason measurement of the number of dystrophin-positive fibers was used as the primary endpoint.
A description of the method was presented at the FDA dystrophin workshop, slides here.
Data:
My Take: They saw a consistent increase in dystrophin-positive fibers in patients at 24 weeks, but not at 12 weeks. Their conclusion was that it takes more than 12 weeks for enough dystrophin to accumulate due to the slow turnover of the protein. The placebo patients crossed over to receive eteplirsen at 24 weeks, and they also saw an increase in dystrophin-positive fibers at 24 weeks after starting treatment. The 30 mg/kg dose group saw a further increase in dystrophin positive fibers from 24 to 48 weeks, suggesting that dystrophin continues to accumulate. The amounts produced appeared similar between the 30 mg/kg group and the 50 mg/kg group. The 4th biopsy may add additional information about to what levels dystrophin is able to be maintained over long time periods of treatment (3+ years). All of the sections were scored by the same person, but the FDA seemed to want multiple people to score the sections & see the concordance rate. I expect this analysis to be part of the rescored biopsies that have been performed.
Immunofluorescence - Mean change in fluorescence intensity:
Method: They also performed analysis on the change in fluorescence intensity from baseline. There are a number of methods for measuring changes in fluorescence intensity that are similar and demonstrated to have good intralab & interlab concordance. The 3 methods used there are similar to each other, but I believe Sarepta (which did its analysis before the paper was published) did their analysis slightly differently than any of those 3 methods. Sarepta's method, to me, seemed most similar to the Taylor method, which creates a spectrin (muscle membrane protein) mask to only count presence of dystrophin at the membrane. Then the dystrophin intensity is taken for the whole image (not for each fiber individually). Sarepta did not appear to use a spectrin mask, but I believe said they used the trace dystrophin staining (or more intense staining in revertant fibers) that outlines the muscle fiber to determine the area to score dystrophin intensity from. In the Taylor paper (above) they found that this method had high concordance with the quantities determined by Western blot, but immunofluorescence was more sensitive at lower levels of dystrophin.
A description of the method was presented at the FDA dystrophin workshop, slides here.
Data:
My Take: While the dystrophin produced was distributed throughout many muscle fibers, as indicated by the increase in dystrophin-positive fibers to about 40% of normal, they only saw increases in total dystrophin intensity to about 15% of normal, which is more representative of the actual amount of dystrophin being made. This still is a significant change from baseline, as indicated by the individual patient data in the 2nd figure. This is also above the 10% threshold suggestive of benefit from the BMD patient data - although that number comes from data measured by the less sensitive Western blot assay.
The individual patient data shown in the bottom figure does raise some questions for me. For instance, they seem to be able to detect significant increases in dystrophin intensity at 12 weeks for some patients, and this wouldn't be as consistent with their dystrophin-positive fiber data that saw no increases at 12 weeks, which they attributed to the slow turnover of dystrophin. Additionally, there is a note at the bottom of the figure that this analysis is based on analysis of 40x magnification of the images, and that 20x magnifications were used at 0, 12, and 24 weeks, but no significant increases were observed. I don't know why one magnification would show increases, but the other wouldn't, and that would seem to be a concern. I would hope in the rescores or the 4th biopsy they follow exactly one of the three methods used in the IF quantification paper mentioned previously, which would give me further confidence in this data.
Western Blot:
Method: A Western blot is one of the most common ways of measuring a protein's presence. It is semi-quantitative, and has been used to measure the relative amounts of dystrophin in DMD vs. BMD vs. normal muscle biopsies. Sarepta has only so far presented Western blot data from a single patient. With a Western blot, the cells from a section of the muscle biopsy are lysed, and their protein contents are run on a gel that separates them by size, before being probed with an antibody to detect the presence, and roughly the quantity, of a given protein.
Data:
My Take: While there is clearly a band indicative of dystropin being produced, this Western is presented in a sloppy fashion. They clearly loaded much less total protein in the control lane from normal muscle, and yet even in the eteplirsen study paper they did not state how much total protein they loaded - my guess is a 10x or 15x dilution. The top row (presumably dystrophin) and bottom row (presumably loading control) aren't even labeled. Also the control sample is not adjacent to the 48 wks Tx sample, it has been placed there, but I assume all the samples were at least run on the same gel, otherwise they really cannot be compared. From this blot Lu et al., who reviewed data from the different exon-skipping trials, guessed that this was definitely not above the 10% threshold of normal dystrophin levels, but it's difficult to tell. If I had to guess, I'd say it's in the 5-10% range, but I expect them to have more thorough Western blot data tomorrow with a dilution series of control muscle (dilute control muscle sample down to 20%, 10%, 5% etc.) to get a better sense of the quantity. Lu et al. consider Western blots to be the preferred, but less sensitive, method of dystrophin detection compared to immunofluorescence - see figure below from Taylor et al. for concordance of WB to IF.
Concordance of Western Blot & Immunofluorescence
From Taylor et al., 2012
#1-5 DMD, 6-7 IMD, 8-12 BMD
One of the discordant points may be due to BMD mutation causing loss of membrane localization & thus low scoring based on immunofluorescence.
RT-PCR:
Method, Data & My Take: Another confirmatory measurement for dystrophin production by exon skipping is RT-PCR. RT-PCR can be used to look at the dystrophin RNA transcript and see if the drug is causing production of a smaller exon 51-skipped RNA. Sarepta has so far only shown data from 1 patient (different patient from the Western blot), but that patient appears to have an increase in the predicted exon 51-skipped RNA. I would like to see this analysis in all patients, if there is sufficient material from biopsies. This data, for me, is the least important measurement quantitatively, as long as they can show some band for the skipped product.
What Others are Saying About the Dystrophin Data:
I also would suggest taking a look at some of the reviews by those in the DMD field that have discussed the dystrophin & clinical trial data from Sarepta, BioMarin/Prosensa, and PTC Therapeutics (ataluren). These have all helped inform my opinion & most of the papers are freely available:
Lu et al.: What Can We Learn From Clinical Trials of Exon Skipping for DMD?
Hoffman & McNally: Exon-skipping therapy: a roadblock, detour, or bump in the road?
Merlini & Sabatelli: Improving clinical trial design for Duchenne muscular dystrophy
Wilton et al.: The emperor's new dystrophin: finding sense in the noise
Kole & Krieg: Exon skipping therapy for Duchenne muscular dystrophy
Conclusions:
The fact that the study was double-blinded at 24 weeks, and those analyzing the dystrophin data were blinded to the identity of the samples at the 48 week time point as well, gives me confidence that they are truly producing dystrophin above the level of detection in these assays. While there is expected to be variability from patient to patient in the amount of dystrophin produced, due to the fact that you are relying on a small biopsy to be representative of the entire muscle, the methods used should be able to detect if a potentially beneficial amount of dystrophin is being produced. The fact that they could measure it by a number of different assays gives me more confidence, and the amounts that they appear to be producing are in the area of what would be expected to be clinically beneficial (although this is still an open question). That is more evidence than can be said for other drugs intended to increase the production of dystrophin, such as drisapersen and ataluren, which have either not attempted or never showed very convincing dystrophin data. Dystrophin is the protein missing in DMD, and I feel it's essential to show production of dystrophin, both to demonstrate the drug is working as expected, as well as to make future clinical development easier. I listed a number of concerns with the different methodologies they used, and perhaps in the past the FDA may have had concerns as well, and I hope, and expect, to see cleaner data tomorrow.
The key things I'll be looking for tomorrow from the dystrophin data are:
Immunohistochemistry - percent-positive fibers: rescores by independent evaluators are similar to the original scores.
Immunofluorescence - intensity: the amounts at 4th biopsy, and did they use a different method for calculating the IF intensity.
Western blot: amount at 4th biopsy, data for more patients, and a dilution series of normal muscle as a control to better determine the amount of protein being produced in treated patients.
RT-PCR: data from more patients.
I wrote a follow-up post here with my thoughts on the updated data.
Disclosure: I own shares of Sarepta
Tuesday, September 8, 2015
Can CD47 blockade help bridge innate and adaptive immunity?
The recent Nature Medicine paper, "CD47 blockade triggers T cell-mediated destruction of immunogenic tumors," suggests that dendritic cells might have a larger than previously appreciated role in the anti-tumor efficacy of CD47 antibodies. Previous work, mostly from the Weissman lab at Stanford, had suggested that macrophages were the predominant effector cell for CD47 antibodies. Macrophages, in general, are the major phagocytic cell in the body, eating pathogens and dead or dying cells. CD47 is thought to serve as a "don't eat me" or anti-phagocytic signal on the surface of cells. CD47 is expressed on normal cells, and its expression is upregulated on blood stem & progenitor cells when they are mobilized to enter the peripheral blood. Specifically, CD47 expression on red blood cells appears to limit their destruction, and a potential toxicity that has emerged from preclinical testing of CD47 antibodies is the depletion of red blood cells, and subsequent anemia.
CD47 has emerged as a potential target in cancer, as it is frequently overexpressed on a variety of tumor types. CD47 is potentially a way cancer cells protect themselves from phagocytosis, since they can have elevated levels of pro-phagocytic signals. Initially found to be upregulated in hematologic malignancies such as AML by the Weissman group (papers here & here), CD47 was subsequently found to also be overexpressed in a wide variety of solid tumors as well. Furthermore, in each of these papers, the Weissman lab demonstrated that an antibody that blocks the interaction between CD47 on tumors & SIRPalpha on macrophages (and other immune cells) has substantial anti-tumor activity against human tumors xenografted into immunodeficient mice. In some additional experiments by the Weissman lab, they found that macrophages, after having phagocytosed cancer cells, could present antigens and prime a T cell response. However, these experiments were primarily done in vitro, not in live animals with an intact immune system.
Here are the things I took away from the paper:
Intratumoral anti-CD47 can cause rejection of immunogenic mouse tumors:
To better understand the effect of CD47 antibodies in the context of a complete immune system, the authors of this paper tested CD47 antibodies in syngeneic mouse models, where mouse tumors could be transplanted into mice with fully intact immune systems. They used two mouse cancer cell lines that were previously characterized as being immunogenic, the A20 (B-cell) & M38 (colon) cancer cell lines. They used a mouse CD47 antibody (MIAP301) to treat these tumors. They started out treating the mice with a systemic dose of 400 ug, which is a similar dose to those used by previous groups. They saw responses, but interestingly then decided "to rule out any effect on peripheral tissues" by injecting a lower dose (50 ug) directly into tumors, which is the regimen they used for the remainder of the paper. With this dosing method, they were able to get substantial anti-tumor activity against established tumors.
Anti-tumor activity of CD47 antibodies is dependent on CD8+ T cells & dendritic cells:
Testing the tumors & treatment regimen in different mouse backgrounds that lack different components of the immune system, the authors found that CD8+ T cells were required for activity, as well as dendritic cells. Specifically, the inflammatory STING pathway in dendritic cells, which senses cytosolic DNA, was necessary for CD47 antibody activity. CD47 antibodies were found to stimulate the dendritic cells to allow them to prime an immune response in endogenous T cells against tumor antigens. Interestingly, depletion of tumor associated macrophages with a
Further, after intratumoral administration of CD47 antibodies, these mice were subsequently able to reject tumors upon rechallenge with tumor cells, suggesting a systemic immunological memory against the tumor cells was established. In the supplemental figures, if they injected the drug at the higher dose & systemically, there was still some efficacy in the absence of T cells. Perhaps the low dose & local administration accounts for some of the discrepancy between the results here versus the human xenograft immunodeficient experiments.
Some additional reasons for the discrepancies between the relative importance of macrophages (previous work) versus dendritic cells (this paper) that were offered:
1. The immunodeficient mice used previously, NSG mice, have a SIRPalpha that can bind human CD47, and thus they might be more susceptible to antibody blockade.
- Not sure what the reasoning is here for why this would be more significant for human CD47 bound to NSG SIRPalpha versus mouse CD47 bound to mouse (BALB/c) SIRPalpha.
2. In xenograft models, the only targets for the human CD47 antibody is CD47 on the human tumor cells, whereas in the syngeneic mouse models, the mouse CD47 antibody can bind to either CD47 on the tumor or on normal mouse tissue that also expresses CD47.
- Trillium actually has data that the "antigen-sink" effect of having target present on both normal and tumor cells doesn't mitigate the efficacy in AML lines xenografted into the NSG mouse, shown below:
Trillium Therapeutics, ASH 2013
Additionally, depending on the levels of CD47 expression and the immunogenicity of the tumor, it's possible that the relative contributions to anti-tumor activity of macrophages, dendritic cells or NK cells may be different between different tumors.
Some experiments I would have liked to see them do within the context of this paper:
1. They don't appear to quantify changes in T cell infiltration or PD-L1 expression changes on tumor from before to after treatment, which may have shown the expected change to a more "T cell-inflamed" microenvironment.
2. I also would have liked to see the induction of a systemic immune response by implanting 2 tumors on either mouse flank and only injecting one, and seeing if the contralateral tumor shrunk in response to CD47 antibody injection. A necessary control would be injecting low dose anti-CD47 into a non-tumor flank with presumably little systemic effects on the contralateral tumor.
Potential combinations:
The necessity of STING for the anti-tumor activity of anti-CD47 antibodies suggests that an intratumoral injection of CD47 antibodies can activate STING. This pathway has garnered some interest by biotech, with Aduro seemingly the furthest advanced in developing STING agonists. They fairly recently partnered this program with Novartis. The hope is that activation of this pathway in innate immune cells in the tumor microenvironment can stimulate the infiltration and activity of T cells. The hypothesis is that PD-1/PD-L1 antibodies work best when there is already an active immune response at the tumor, that is being suppressed by those pathways. There are other tumors that lack this immunophenotype and are characterized as non-T cell-inflamed. This is illustrated in the figures from a review by Thomas Gajewski and colleagues:
 |
| T cell-inflamed microenvironment |
 |
| Non-T cell-inflamed microenvironment |
Now, there is a difference between STING being required for the activity of CD47 antibodies versus something being a direct STING agonist. For instance, the CD47 antibodies are probably much more dependent on tumor CD47 levels for their ability to cause all their potential downstream effects. We don't know the amount of STING activation with anti-CD47 antibodies versus direct STING activation, or how differences inherent to a specific tumor or its microenvironment would affect the indirect apparent STING activation after CD47 antibody treatment. However, I would imagine that this suggests CD47 antibodies could rationally be combined with PD-1/PD-L1 blockade. As mentioned earlier, it would be interesting to see how intratumoral anti-CD47 affects T cell infiltration. The Weissman lab did use one syngeneic model in Figure 6 of this paper from Willingham et al., and found that there was increased T cell infiltration after treatment, although this was not quantified.
Conclusions:
This paper demonstrated that CD47 antibodies can potentially stimulate a CD8+ T cell adaptive immune response, and this is largely dependent on dendritic cells. While this is somewhat at odds over which innate immune cell type is critical for the cross-priming activity, both this group and the Weissman group have shown these antibodies have the potential to induce an adaptive immune response, and this is in addition to any direct effects through phagocytosis or ADCC. The dependence of the anti-tumor activity on T cells and the STING pathway in dendritic cells is of interest, as it suggests this therapeutic approach may make for a good combination with immune checkpoint blockade. One final point is that intratumoral administration of anti-CD47 could induce these effects. This could potentially be a different route of administration, and while the previously mentioned paper from Willingham et al. found that intratumoral administration still led to systemic exposure, the lower doses used in this paper maybe could limit this. Perhaps this could somewhat mitigate the potential for systemic toxicity (against red blood cells, for instance), that might be dose-limiting, as CD47 antibodies are being developed in the clinic. While this paper presents preclinical data (like all the other current data on CD47-targeted therapeutics), it does add useful information about how this approach may also stimulate an adaptive immune response against tumors and could inform their clinical development.
Edit: DrX published a comment below that brings up some points about the methodology of the paper for distinguishing the effect of macrophages and dendritic cells that is worth checking out.
Disclosure: I own shares of Trillium Therapeutics and Aduro Biotech
Subscribe to:
Posts (Atom)